Lab-grown meat just took a huge step toward feeling like real meat
Harvard researchers have made a discovery.
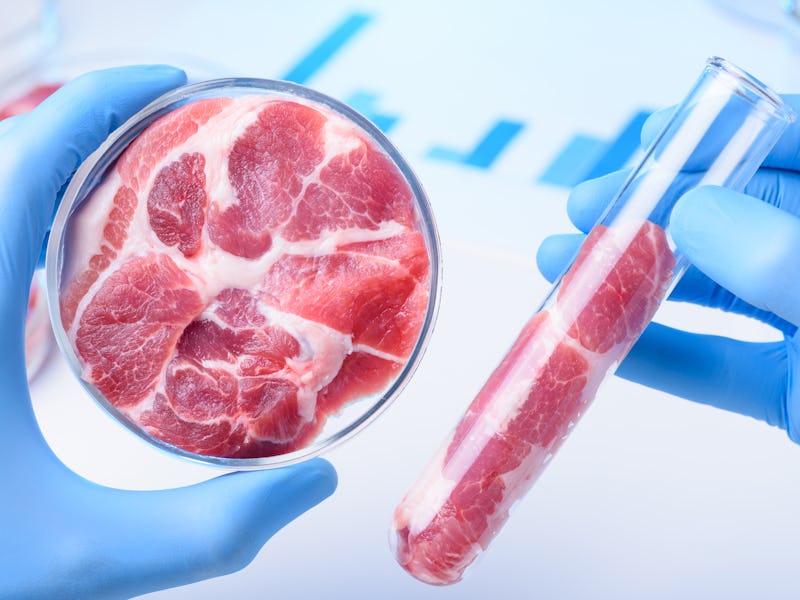
Lab-grown meat is coming to your plate, and thanks to a new breakthrough in its development, it may feel like traditional meat.
Researchers at Harvard University have discovered a way to use edible gelatin scaffolds to mimic real meat’s texture and consistency. The team used cow and rabbit muscle cells on the scaffolds to recreate the long, thin fibers that give meat its distinctive feel.
The research was published Monday in the journal NPJ Science of Food. Kit Parker, senior author of the study, said in a statement that he first started thinking about the idea after being a judge on the Food Network.
“The materials-science expertise of the chefs was impressive,” said Parker. “After discussions with them, I began to wonder if we could apply all that we knew about regenerative medicine to the design of synthetic foods.”
It turns out there’s a lot that can be learned from regenerative medicine.
The world's first lab-grown burger, as held by Mark Post.
Lab-grown meat: how it’s gradually coming together
Lab-grown meat is a developing area of food that could offer an alternative to traditional farm-sourced meat. The technology involves taking animal stem cells and placing them in a nutrient-rich environment, encouraging them to grow out further.
The first lab-grown burger eaten in public was created by Mark Post and consumed in 2013, and it was described as one of the first tasters as similar to a McDonald’s burger. Companies that have sprung up in the area predict lab meat could hit plates in 2021. Aleph Farms, one of the firms in the field, is growing four different petri dishes of cells to try and recreate more advanced meats like steak.
Parker, one of the authors of the Harvard study, started thinking about how the lessons learned in regenerative medicine could apply to food. After all, both require knowledge of how to build healthy cells and scaffolds. The idea started developing to use a “scaffold” material to help muscle meat grow in three dimensions. These adherent cells need something to hold as they develop.
The idea was developed to use food-safe gelatin fibers that could mimic the matrix that holds muscle cells in place. This scaffold was developed into nanofibers using a technique called immersion rotary jet-spinning. These fibers were then seeded with cow and rabbit muscle cells to encourage them to grow along the structures.
“This is our first effort to bring hardcore engineering design and scalable manufacturing to the creation of food,” said Parker.
Peeling apart gelatin fibers.
How did the end product compare to the original meat? It may need some work before it’s indistinguishable. The final product had a similar texture to the original, but traditional meat packed more muscle fibers, hinting at its maturity.
“Muscle and fat cell maturation in vitro are still a really big challenge that will take a combination of advanced stem cell sources, serum-free culture media formulations, edible scaffolds such as ours, as well as advances in bioreactor culture methods to overcome,” Luke MacQueen, another author of the study, said in a statement.
It’s precisely innovations like these that could suggest a bright future for lab meat. A study by consultancy firm AT Kearney predicted in June 2019 that lab-grown meat will overtake the likes of plant-based burgers like Impossible Foods and account for 40 percent of the market by 2040. This, the firm reasoned, is in part because the nascent technology offers more scope for innovations that closely match the taste of meat compared to plant-based food.
It may not be long before realistic lab meat makes a big splash.
Read the abstract below:
Bioprocessing applications that derive meat products from animal cell cultures require food-safe culture substrates that support volumetric expansion and maturation of adherent muscle cells. Here we demonstrate scalable production of microfibrous gelatin that supports cultured adherent muscle cells derived from cow and rabbit. As gelatin is a natural component of meat, resulting from collagen denaturation during processing and cooking, our extruded gelatin microfibers recapitulated structural and biochemical features of natural muscle tissues. Using immersion rotary jet spinning, a dry-jet wet-spinning process, we produced gelatin fibers at high rates (~ 100 g/h, dry weight) and, depending on process conditions, we tuned fiber diameters between ~ 1.3 ± 0.1 μm (mean ± SEM) and 8.7 ± 1.4 μm (mean ± SEM), which are comparable to natural collagen fibers. To inhibit fiber degradation during cell culture, we crosslinked them either chemically or by co-spinning gelatin with a microbial crosslinking enzyme. To produce meat analogs, we cultured bovine aortic smooth muscle cells and rabbit skeletal muscle myoblasts in gelatin fiber scaffolds, then used immunohistochemical staining to verify that both cell types attached to gelatin fibers and proliferated in scaffold volumes. Short-length gelatin fibers promoted cell aggregation, whereas long fibers promoted aligned muscle tissue formation. Histology, scanning electron microscopy, and mechanical testing demonstrated that cultured muscle lacked the mature contractile architecture observed in natural muscle but recapitulated some of the structural and mechanical features measured in meat products.