Bones may be the future of battery design
Scientists are turning to an unlikely source of inspiration for next-gen batteries.
The lithium battery has long been the king of electronics. It powers everything from smartphones to self-driving cars — but it also has a serious problem with overheating and, at times, exploding.
Over the past decade, in an effort to develop a safer and more efficient option, scientists have avidly worked toward developing an alternative to lithium: the sodium battery. But there's one problem, cathodes — the electrodes which carry electrons through a battery — for sodium batteries are notoriously unstable.
To remedy this chemical problem, a team of scientists has turned to a biological solution in the form of bones. By creating cathodes that mimic mammal bones in their spongy interior and tough exterior, scientists may have finally found a way to design effective sodium cathodes.
In a proof-of-concept study published Tuesday in the journal Applied Physics Reviews, a sodium battery of this design was able to maintain 91 percent of its capacity for over 10,000 charge cycles.
The need for a new battery — In addition to its rather explosive downsides, mining lithium is increasingly becoming a less viable and environmentally damaging practice as a whole. Salt, on the other hand, is abundant across the globe and much more easily accessible. But while this solution looks good on paper, sodium battery designs have historically suffered from one major flaw: they're difficult to keep stable under high-voltages.
As a solution, Ho Seok Park, a co-author of the paper and chemical engineer at Sungkyunkwan University, and his team turned their attention to another abundant natural resource: the human body.
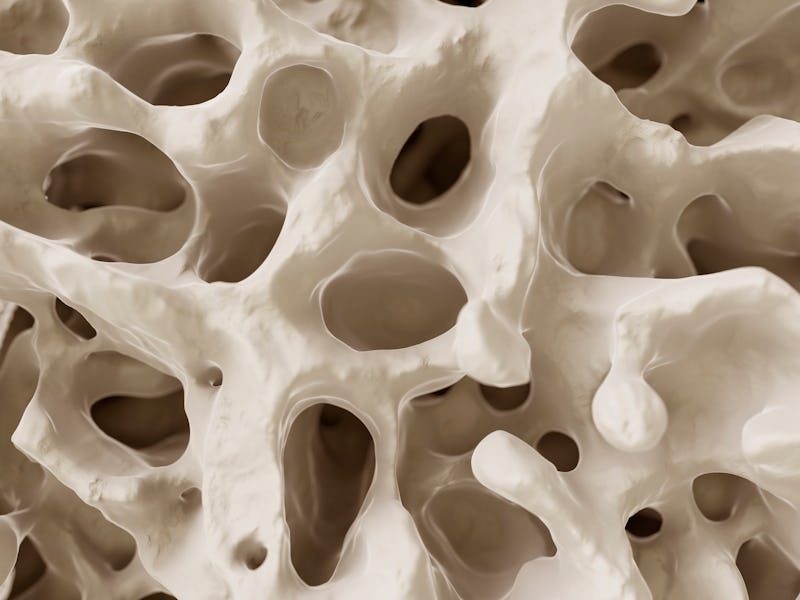
More specifically, the internal structure of mammal bones. As in many other cases, nature often does it best.
"We believe that nature provides a very promising solution to resolve technical problems," said Park. "Accordingly, we tried to find the ideal architecture that can resolve these kinetic and stability limitations."
What's in a bone — Part of what makes mammal bones so appealing as a model for battery cathodes is their dual texture, the study team explains. The outside of bones is composed of hard, mostly inflexible calcium, but the interior of the bone — the marrow — is much spongier and far more flexible.
In some restaurants marrow is a delicacy, but to scientists it was a important inspiration.
"The spongy bone possesses a surface area ten times higher than the compact bone," the authors write. "This creates the classic soft-hard composite effect, allowing the bone to flex under stress and yet structurally support the load of the body."
Structural integrity like this is precisely what sodium batteries lack.
A bone-like battery — Instead of bone, the researchers created their biomimetic battery using a porous system of elements called NVP to mimic bone marrow and a dense shell of graphene oxide to mimic bone's tough calcium exterior.
When testing the stability of their new cathode, the team saw impressive stability gains and overall lifecycle extension for the battery. The battery cell was capable of charging to ultrahigh rates in just 3.6 seconds and could maintain 91 percent of its capacity after 10,000 charging cycles. For comparison, lithium batteries typically last only between 400 and 1,200 cycles.
What's next — Despite this design's initial success, the researchers stress that is still only a proof-of-concept design. In order to demonstrate the long-term success of this model, Park says that a larger-scale synthesis of this design will be necessary to see how it will fair in more practical applications — this study's trials, for example, were done at a toasty 122 degrees Fahrenheit.
Nevertheless, the researchers hold out hope that their design could play an important role in finally dethroning the lithium battery.
Abstract: Sodium ion batteries are an emerging candidate to replace lithium ion batteries in large-scale electrical energy storage systems due to the abundance and widespread distribution of sodium. Despite the growing interest, the development of high-performance sodium cathode materials remains a challenge. In particular, polyanionic compounds are considered as a strong cathode candidate owing to their better cycling stability, a flatter voltage profile, and stronger thermal stability compared to other cathode materials. Here, we report the rational design of a biomimetic bone-inspired polyanionic Na3V2(PO4)3-reduced graphene oxide composite (BINVP) cathode which achieves ultrahigh rate charging and ultralong cycling life in a sodium ion battery. At a charging rate of 1C, BI-NVP delivers 97% of its theoretical capacity and is able to retain a voltage plateau even at the ultra-high rate of 200C. It also shows long cycling life with capacity retention of 91% after 10,000 cycles at 50C. The sodium ion battery cells with a BI-NVP cathode and Na metal anode were able to deliver a maximum specific energy of 350 W h kg-1 and maximum specific power of 154 kW kg-1. In-situ and post-mortem analyses of cycled BI-NVP, including by Raman and XRD spectra, HRTEM and STEM-EELS, indicate highly reversible dilation – contraction, negligible electrode pulverization, and a stable NVPrGO layer interface. The results presented here provide a rational and biomimetic material design into the electrode architecture for ultrahigh power and ultralong cyclability of the sodium ion battery full cells when paired with a sodium metal anode.