These Worms Abandoned Sex to Improve Their Species
Now their biology is more diverse.
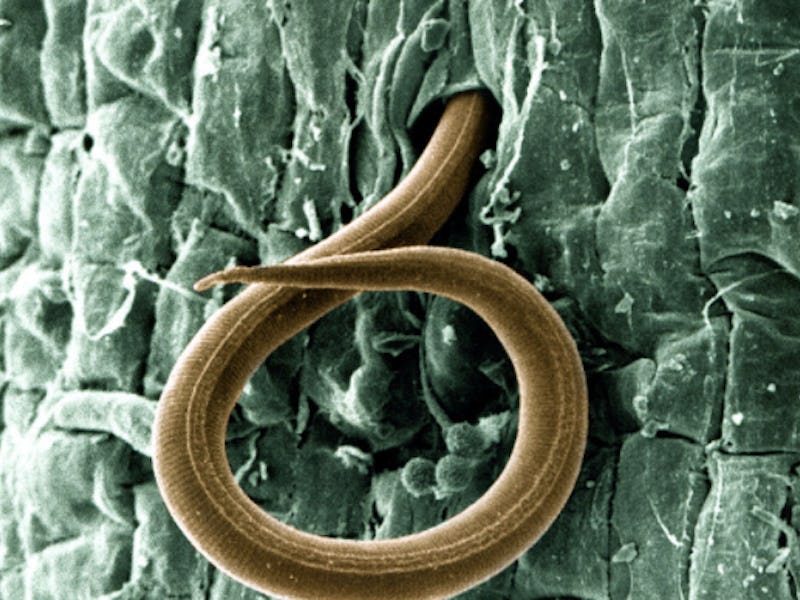
There’s a new reason to abstain from sex — it might actually benefit the species.
It might sound counterintuitive, but according to a new study published Thursday in PLOS Genetics, it’s true, at least for parasitic worms.
Although nearly all animals reproduce sexually, there’s a small percentage that’s managed to procreate without sex and the root-knot nematode is one of them. The microscopic plant pest feeds on roots and has been known to have a devastating effect on food crops across many different climates.
Scientists had in the past considered non-sexual reproduction to be a counter-evolutionary tactic; as sexually reproductive species tended to outpace their sexless counterparts, often leading to an evolutionary dead end. Not so with the nematode though, who’ve not only managed to begin reproducing without sex, they’ve also become a more devastating agricultural parasite than their sexually-reproducing cousins.
So what makes the nematode the exception to the rule? It’s all in the genes. By comparing the genomes of the most devastating species of asexual nematodes to sexual nematodes, a group of researchers at the French National Institute for Agricultural Research determined that the asexual nematodes were undergoing a state of hybridization that was making their genome architecture incredibly diverse. The asexual nematodes are polyploid, meaning their cells are made up of more than the usual two chromosomes (one from each parent), like most animals.
“We hypothesize that their peculiar hybrid genome structures provide these nematodes with a potential for adaptation and plasticity and could explain their paradoxical success in the absence of sex,” the authors of the study say.
Basically, this genome diversity has made the asexual nematode extremely adaptable to different environments and plant hosts, allowing them to beat out their sexual counterparts.
The findings could help scientists figure out ways to combat the spread of nematodes and in doing so preserve food crops.
Who knows, maybe if humans could figure out how to reproduce asexually like the nematode, maybe we could adapt to the extreme conditions that will inevitably come with climate change. But then we would be living on a barren planet and having no sex…
Plos Genetics, one of a number of open-access journals spearheaded by the Public Library of Science, publishes original research, essays, interviews and viewpoints covering all areas of biology.
Abstract:
Root-knot nematodes (genus Meloidogyne) exhibit a diversity of reproductive modes ranging from obligatory sexual to fully asexual reproduction. Intriguingly, the most widespread and devastating species to global agriculture are those that reproduce asexually, without meiosis. To disentangle this surprising parasitic success despite the absence of sex and genetic exchanges, we have sequenced and assembled the genomes of three obligatory ameiotic and asexual Meloidogyne. We have compared them to those of relatives able to perform meiosis and sexual reproduction. We show that the genomes of ameiotic asexual Meloidogyne are large, polyploid and made of duplicated regions with a high within-species average nucleotide divergence of ~8%. Phylogenomic analysis of the genes present in these duplicated regions suggests that they originated from multiple hybridization events and are thus homoeologs. We found that up to 22% of homoeologous gene pairs were under positive selection and these genes covered a wide spectrum of predicted functional categories. To biologically assess functional divergence, we compared expression patterns of homoeologous gene pairs across developmental life stages using an RNAseq approach in the most economically important asexually-reproducing nematode. We showed that >60% of homoeologous gene pairs display diverged expression patterns. These results suggest a substantial functional impact of the genome structure. Contrasting with high within-species nuclear genome divergence, mitochondrial genome divergence between the three ameiotic asexuals was very low, signifying that these putative hybrids share a recent common maternal ancestor. Transposable elements (TE) cover a ~1.7 times higher proportion of the genomes of the ameiotic asexual Meloidogyne compared to the sexual relative and might also participate in their plasticity. The intriguing parasitic success of asexually-reproducing Meloidogyne species could be partly explained by their TE-rich composite genomes, resulting from allopolyploidization events, and promoting plasticity and functional divergence between gene copies in the absence of sex and meiosis.